Si-Click® – (Copper-free) Click-Chemistry
Click chemistry is a class of chemical reactions between 2 reactive compounds that occur with high efficiency and specificity, without the need for special solvents or even catalysts.
This characteristic can be used very well for labeling proteins in intact cells: Mounting labels on proteins allows observing and tracking them in their natural environment and represent a powerful tool for studying cell biology and even for developing drugs. Nowadays, a large variety of approaches exists for protein labeling – differing in size of modification, general scope, as well as the ease of usability and exchangeability.
A major drawback of many genetically encoded tags represents their large size which can be overcome by making use of the fascinating potential of specifically labeling single amino acid residues with small molecule dyes. Genetically encodable unnatural amino acids (UAAs) allowing for biocompatible and even bioorthogonal site-specific labeling of proteins with (fluorogenic) dyes are under deveolopment.
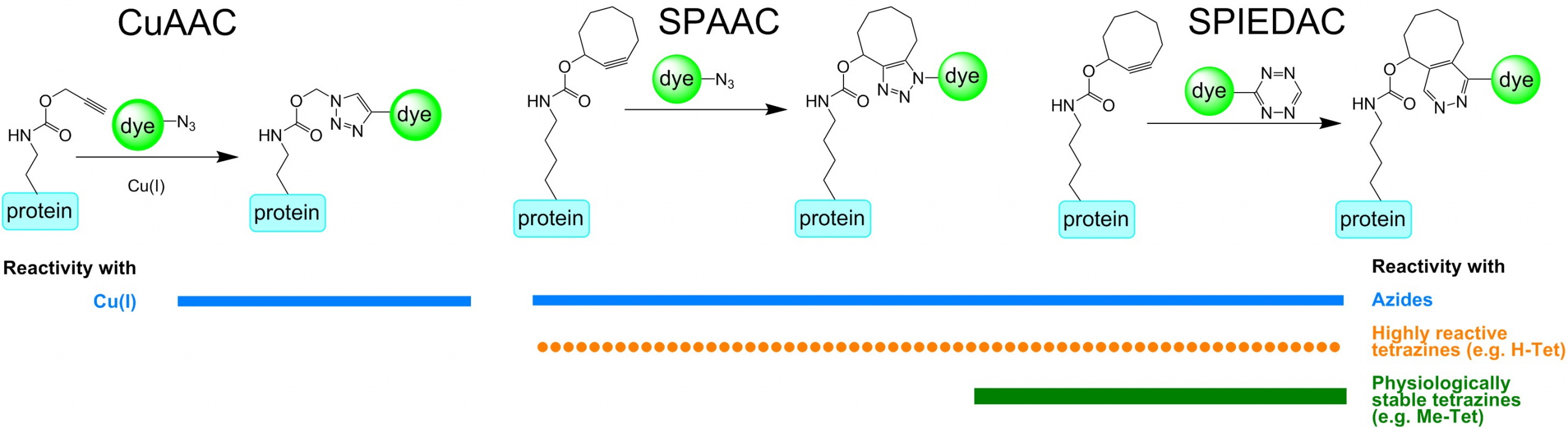
The new lysine-based compounds have (bi)cyclooctyne (e.g. SCO / BCN), trans-cyclooctene (e.g. TCO-4-en / TCO*) or propargyl units (e.g. PrK) that can be grafted into proteins – amongst other of E. coli and mammalian cells. Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC) click chemistry between azides and linear alkynes as well as the two most potent representatives of in vivo bioorthogonal chemical reactions, strain-promoted alkyne-azide cycloaddition (SPAAC) and strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC), can be used to efficiently and rapidly attach fluorophores conjugated to azide and tetrazine moieties, respectively. Due to the modularity of the technique, any imaginable probe fused to azide or tetrazine can be attached to any protein modified with a corresponding unnatural side chain with the maximum freedom of placement within the protein under investigation.
The strained rings (cyclooctin, transcyclooctene) are also used for the triggered release of APIs: The click reaction and thus the selected cleavage of tumor-bound antibody drug conjugates (ADCs) can be a powerful tool in ADC therapy.
Another application of the click reagents is in surface plasmon resonance (SPR) – a widely used method for characterizing bindings, e.g. between small molecules and target proteins. Here, the sensors and the ligands (e.g. proteins) are tagged with appropriate click tools and can thus interact quantitatively and extremely selectively.
Relevant tools you will find here: SiChem_PEG-tools or in our Shop
…when larger quantities are required
SiChem develops customized click amino acids with different substituents and (PEG) linkers or dyes according to your specifications. Quantities in kg scale can be produced in high purity in our fully equipped laboratories.
Also corresponding precursors, such as various active esters and alcohols with strained ring-systems can be synthesized in larger quantities.