Nomenclature of the Inositol Phosphates
Nomenclature
Inositols belong to the cyclitols. The formula is C6H12O6 and the structure is a cyclohexane ring with a hydroxy group attached to each carbon. The group of inositols consists of nine isomers which differ with respect configuration at the carbons. Each isomer is named with a distinct prefix. The (+) and (-)-chiro-inositols have two axial hydroxy groups, are optically active and form the only pair of enantiomers. The remaining seven isomers have one more planes of symmetry and are hence meso compounds. The scyllo isomer has no, myo has one, neo and epi have two, and the cis, muco, and allo isomers have three axial hydroxy groups.
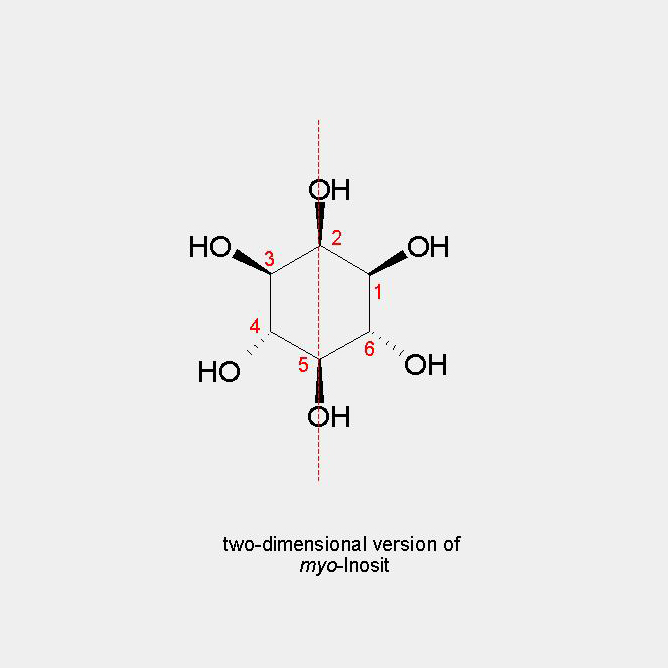
The by far most common isomer in nature is myo-inositol. Per UIPAC definition is the carbon carrying the single axial hydroxy group numbered with 2. If one imagines that this axial group is the head of a turtle, the right front foot of the turtle is always called 1 and the remaining numbers are distributed counter-clockwise. Through carbons 2 and 5 lies a symmetry plane. Accordingly, substitutions at the hydroxy groups or the carbons 2 and 5 will maintain the meso-character. This is also true, if the hydroxy groups or carbons 1 and 3, or 4 and 6 are substituted evenly. Any other modification will result in pairs of enantiomers. In the literature offen the terms of D- or L-enantiomers are used. For simplicity this should be avoided in the future.
two-dimensional representation of myo-Inositol
The group of inositols consists of nine isomers which differ with respect configuration at the carbons. Each isomer is named with a distinct prefix. The (+) and (-)-chiro-inositols have two axial hydroxy groups, are optically active and form the only pair of enantiomers. The remaining seven isomers have one more planes of symmetry and are hence meso compounds. The scyllo isomer has no, myo has one, neo and epi have two, and the cis, muco, and allo isomers have three axial hydroxy groups.
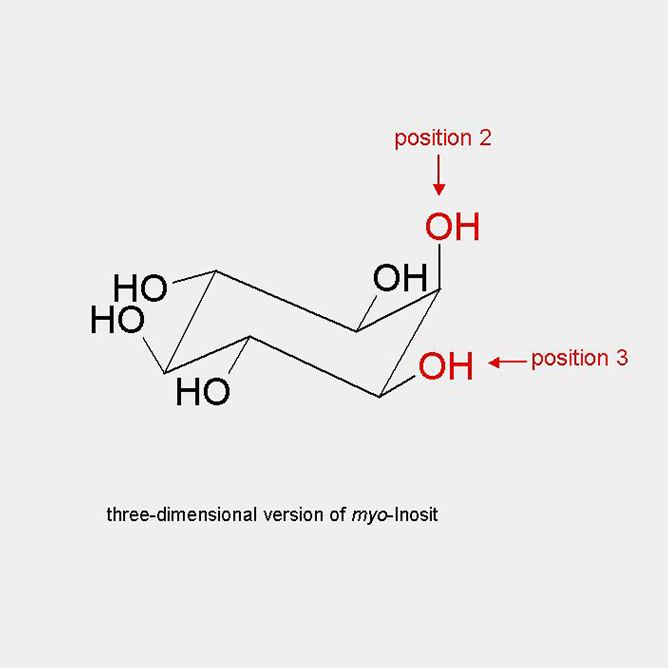
three-dimensional representation of myo-Inositol
The by far most common isomer in nature is myo-inositol. Per UIPAC definition is the carbon carrying the single axial hydroxy group numbered with 2. If one imagines that this axial group is the head of a turtle, the right front foot of the turtle is always called 1 and the remaining numbers are distributed counter-clockwise. Through carbons 2 and 5 lies a symmetry plane. Accordingly, substitutions at the hydroxy groups or the carbons 2 and 5 will maintain the meso-character. This is also true, if the hydroxy groups or carbons 1 and 3, or 4 and 6 are substituted evenly.
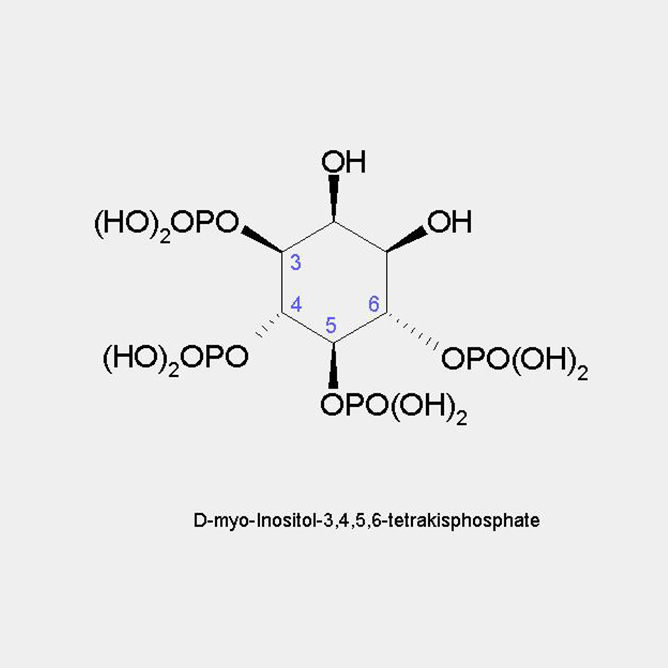
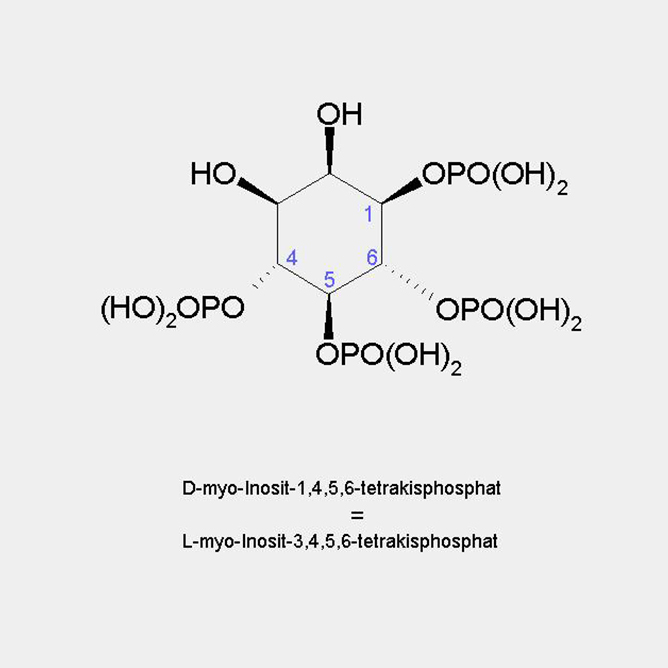
D-myo-Inositol-3,4,5,6-tetrakisphosphate
Any other modification will result in pairs of enantiomers. In the literature offen the terms of D- or L-enantiomers are used. For simplicity this should be avoided in the future.
D-myo-Inositol-1,4,5,6-tetrakisphosphate
=
L-myo-Inositol-3,4,5,6-tetrakisphosphate