Technology
The ProDrug Concept
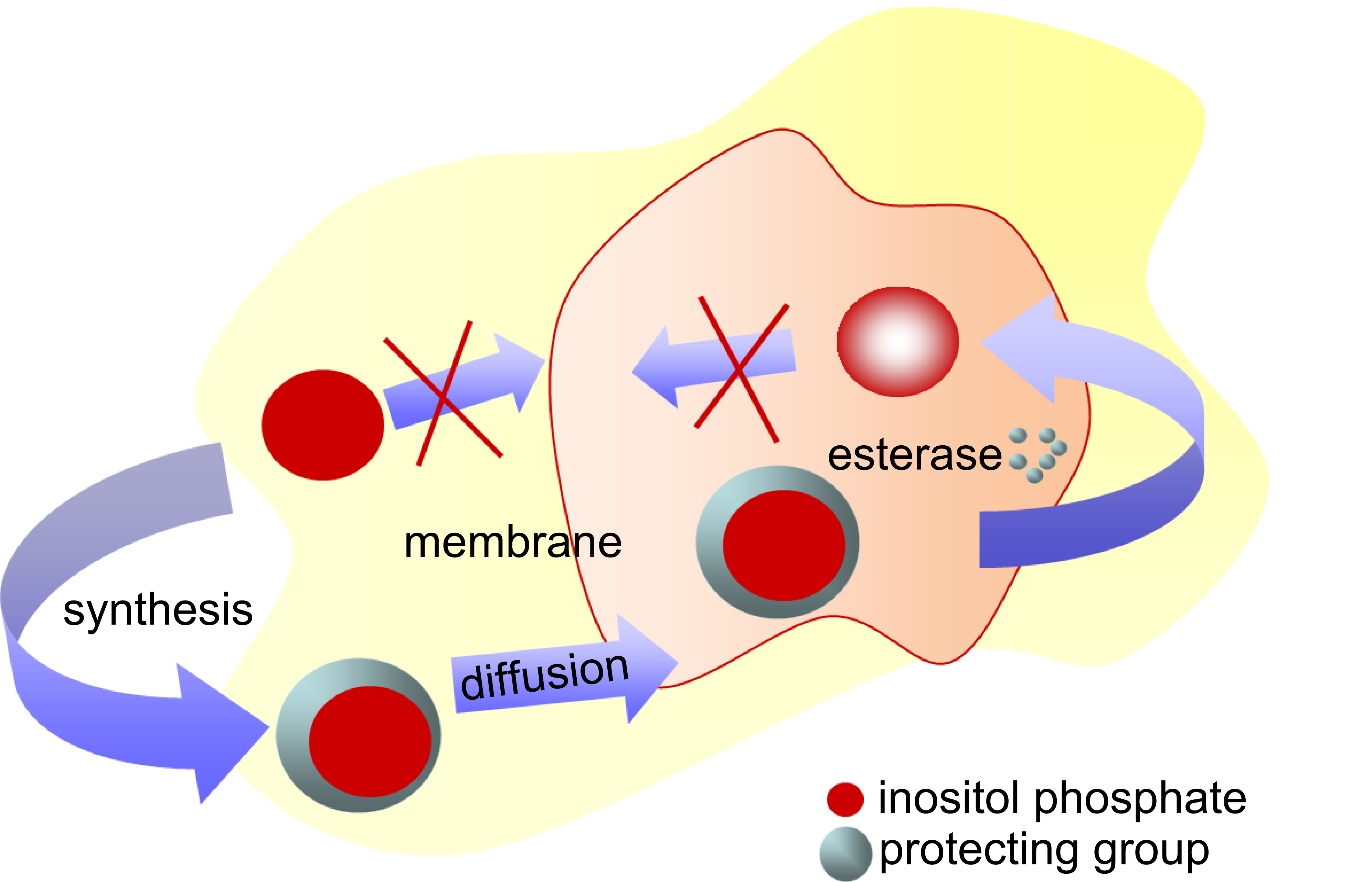
For many of the known small intracellular messengers phosphate groups are characteristic, for instance the inositol phosphates. Under physiological conditions the phosphate groups are partially ionized rendering the molecules sufficiently hydrophilic to prevent them from leaking out of the cell they are generated in. This fact is an important pre-requisite to permit individual responses of single cells to external stimuli. Unfortunately, the same principle applies when external doses of messenger molecules are applied to cells in order to artificially stimulate signaling pathways. These experiments scientists would love to do without disrupting the plasma membrane, as it is unavoidable when electroporation, saponification or microinjection techniques are used.
To circumvent this problem, unpolar masking group are frequently employed in modern drug development. These groups should be stable in the extracellular space but have to be designed in a way that endogenous enzymes inside the cell deprotect the charged groups. This approach is usually called a prodrug approach in Medicinal Chemistry. A large amount of work has been done to increase the efficacy and bioavailability of antiviral drugs (e.g. AZT) or some penicillins. Membrane-permeant derivatives of cyclic nucleotide signaling molecule like cAMP and cGMP showed enormous increases in efficacy over the charged molecules and these compounds became commercially available recently.
For inositol polyphosphates the task appeared to be particularly challenging, due to the relative large number of phosphate groups and the neighboring vicinal hydroxy groups. Scientists from the groups of Roger Tsien and Carsten Schultz were successful in masking the phosphates of many inositol polyphosphates and phosphoinositides with acyloxymethyl ester groups and the hydroxy groups with butyrates. The resulting fully protected derivatives were able to readily penetrate plasma membranes and were shown to generate the original molecules inside cells within minutes after administering the compounds. The acyloxymethyl esters are supposed to be cleaved much more readily than the butyrates and this should prevent scrambling of the phosphates. The lipophilicity of the phosphate masking group determines the overall lipophilicity of the prodrug molecule. Higher membrane penetration and loss in solubility have to be considered by chosing the optimal masking group. If there are already highly lipophilic groups in the molecule as is the case in phospholipids, acetoxymethyl esters on the phosphates will suffice. For the unsubstituted inositol polyphosphates more lipophilic groups like propionoxymethyl ester groups showed increased cellular responses in several studies.
For more details, please refer to:
C. Schultz, Prodrugs of Biologically Active Phosphate Esters.
Bioorg. & Med. Chem. 11, 885-898 (2003)